We received a bunch of questions that we did not have time to answer during the webinar. So we thought you might like to see them answered here.
If you did not get a chance to see the Webinar, or if you just want to watch it again here you go:
What solution should be use for cleaning torches, spray chambers, etc.? What should the acid concentration be?
Autumn: We typically use 25% v/v RBS-25TM for soaking spray chambers or torches, but we also sometimes use 50% v/v HNO3 for temporary soaking (an hour or two) of torches that are particularly “dirty” with visual residue build up. You can also contact the instrument manufacturer or instrument manual for torch maintenance tips based on particular sample types.
Do you have advice for calibration curve troubleshooting?
Autumn: Make sure you are working within the linear range for that element/wavelength and that your low standards are above the detection limit. Depending on your instrument/method, a parabolic rational fit may work better for wider calibration ranges. Ensure that your blank is clean and does not contain the analytes of interest as contaminants resulting in a low bias for those analytes at low levels. Go through and look at the spectra to ensure peaks are centered properly and background points are set correctly.
Sergei: Looking at the actual raw intensities is the key to troubleshooting the calibration curve issues. Especially when evaluating the purity of the Cal. Std. 0 which is referred to as a Blank. The Calibration Curve can be “fine-tuned” by changing the statistical weight of the individual standards (points of the curve). For example, “forcing” the Curve through 0 point by applying a higher statistical weight while looking at the Relative Difference in % on each point which was shown at the last slide of the presentation.
Do you have a training specific to ICP-MS?
Autumn: We don’t currently have a training specific to ICP-MS only, most of our ICP training/technical resources cover both ICP-OES and ICP-MS. However, we do cover ICP-MS related topics in depth at our ICP Conferences (coming up Dec4th-5th and 7th-8th). Our IV Ignite on-demand learning platform, which is launching soon, also offers ICP-MS specific Learning Paths and courses for ICP-MS users at all levels and across a diverse range of industries. Sign up for our newsletter, Ignite Insights for updates.
I run 3 readings and take an average. Why is my first reading lower than the next 2 readings?
Autumn: You probably need to increase your stabilization time to allow time for the sample to reach the plasma and for the signal to stabilize. If it is consistently the first reading only that is low, this will likely be the quickest fix for your issue without having to adjust other instrument parameters that may affect other aspects of the analysis.
I work with a fairly saline matrix, geothermal fluid. We use ceramic accessories due to the characteristics of the sample. How could we evaluate the low precision in the mist chamber and the rest of the accessories?
Autumn: You could look at the mist coming from the nebulizer while the pump is on, but the nebulizer is not inserted in the spray chamber (see example slide for this with video from the presentation). You would be able to see if the mist is forming properly and is of acceptable density, consistent particle size, and consistent flow. You could also try just cleaning the nebulizer by flushing or back flushing with a cleaning solution (like 2.5% RBS-25 or similar) or just water or dilute acid and then checking to see if precision is improved after.
What are the best ways to avoid nebulizer clogging?
Sergei: The best way – switch to a Nebulizer that is designed to prevent clogging. We specialize in designing and manufacturing such nebulizers. You are welcome to get in touch with our team and we will help with a switch. For current nebulizer: If the sample channel clogs – then filtering and keeping the samples in the special enclosure are general recommendations from the ICP manufacturers. If the gas channel clogs, which is indicated by higher nebulizer backpressure, then adding an online particle filter to the Nebulizer Gas supply line AND Argon Humidifier is recommended. Both products are available from TSP.
Autumn: Use an argon humidifier for the nebulizer flow gas. This will help prevent salting out of high TDS samples. You could also increase dilution of the samples and/or filter samples prior to introducing to the instrument. Also ensure you are cleaning your nebulizer frequently, especially if you are running high TDS samples. Nebulizers can be soaked in dilute acid or cleaning solution if there is a clog that won’t come out, but never put nebulizers in an ultrasonic bath as this can damage them!
How often should we change injectors if our ICP-OES is running 18-20 hours per day with high Na concentration?
Autumn: Always examine the injector and torch components for residue buildup or deposits and wear of the injector tip surface – as was shown in the presentation. I would just start doing this daily and make a note in the maintenance log for the instrument of when the injector begins to show any sort of buildup and set up a schedule for cleaning the injector based on that. An argon humidifier will also help to cut down on the salt deposits observed from high TDS samples.
What is the purpose of a carrier solution in ICP-MS? Is it vital and what are typical solutions?
Autumn: The carrier solution pushes the sample out of the sample loop and into the nebulizer and is also used to clean the sample loop between samples.
What is the advantage or is there an advantage of using Argon versus other types of gases?
Sergei: There are both advantages and disadvantages. For example, Nitrogen is much less expensive than Argon but requires much higher RF power to maintain a stable plasma. Helium plasma offers higher degree of ionization for analytes with higher ionization potential, but has lower gas temperature, electron density, and higher cost than Argon. Here is a good reference if you want to dig further into this very interesting topic: Inductively Coupled Plasmas in Analytical Atomic Spectrometry, Akbar Montaser, D. W. Golightly. John Wiley & Sons, 2nd Edition. 1996.
What does Hg realign for?
Sergei: If understood correctly, Hg source is used on some ICPs to generate a reference emission line used for wavelength calibration.
What recommendation will you give to an analysis of Mehlich-3 in soil that gives far values of Mn, Fe, and P in terms of accuracy?
Sergei: We have many customers running analysis of Mehlich-3 extracts in soils.
There should not be any difference to get accurate results for Mn, Fe and P as compared to the other elements typically looked for in this application.
We formulate custom standards in Mehlich-3 matrix, manufactured by Inorganic Ventures, to help analysts check on the accuracy of the results produced by ICP.
Most likely, the difference in values may be related to the extraction procedure rather than to the ICP part of the analysis. Checking with independent fully matrix matched custom standard in Mehlich-3 will help to identify the source of the problem.
I’m working with Tallow. How do I pick the correct method?
Sergei: It seems that tallow is waxy white fat that consists of esters of a few organic acids. If digested – then this organic sample will turn inorganic (aqueous).
We are looking to add 009-63 to our scope in California. We only have one ICP-OES. Any recommendations for successfully going between the normal acidic water samples for our typical metals to analyzing oil? We are already planning on separate sample probes, tubing, etc. but wondered if there is anyone out there who does both on the same instrument?
Sergei: Many of our customers do the switch between aqueous and organic samples. In addition to the autosampler probes, a totally separate set of sample introduction components (nebulizer, spray chamber, torch) dedicated to the organic matrix is almost a must. Plus – different pump tubing material that is resistant to organic solvent.
Any advice for troubleshooting low concentration stability issues on low mass elements like Beryllium?
Sergei: Getting stability at the low concentration that is close to detection limits is challenging in general, regardless of the mass range. Low mass range is just more difficult than the high mass range. Trying different internal standards (Li7 for example) and optimizing the ICP-MS to favor low mass range by increasing the Nebulizer flow might be worth trying.
How do you assess Y bullet position when instrument has plates and not the coil like some PerkinElmer ICP-OES.
Sergei: Perkin Elmer folks should be able to provide the guidance. If not, please let me know and I will reach out to my connections at PE.
I analyze Titanium Allor (Al8%-V4%), digestion with HCL-HF-HNO3. It’s very difficult to achieve the right result on my CRM for V and Al. Calibration for Al: 200ppm to 600ppm and V 100-400ppm 4-point on curve (curve looks okay). Introduction system problem?
Sergei: We have a few customers running the analysis of very similar alloys. The key might be in the composition of your calibration standards. We have designed custom standards for this application manufactured by Inorganic Ventures.
We have experienced torch melting couple of times. What could be root causes of this? And maybe possible solutions?
Sergei: Melting of the torch does happen sometimes. Most often, during the ignition sequence. If that is the case, please monitor the process and if you see if something did not go normally, shut down the ignition process, flush the torch by Argon for a couple of minutes, and try again. If it happens during the run, we need to look deeper on what is going on.
Autumn: Torch position could be incorrect and could be too close to the OPI. Adjust the torch where the inner tube opening is ~2-3 mm behind the first coil. Also, for some instruments, you may need to make sure the instrument is always aspirating a solution while the plasma is running and don’t allow the instrument
to run dry (i.e. autosampler probe in the up position). Set the autosampler to return to the rinse station after the analysis finishes.
What about precision on dilution?
Autumn: We always recommend gravimetric preps (prepping by weight) for all standards and samples. This will greatly improve accuracy and precision over volumetric preps. Diluent for your standards should be the same diluent used for the samples you are analyzing.
If you observe condensation (droplets) on the tubing that connects the gas humidifier to the remainder of the sample introduction tubing, is that an issue of precision?
Autumn: If droplet formation is observed on the tubing, this could be an indication that the tubing is dirty and needs to be replaced. Also ensure that the humidifier isn’t over filled with DI water and that the connections are properly installed.
Sergei: Yes, moisture accumulation in the tubing coming out of the Argon Humidifier can degrade the precision.
How can we pick wavelengths for a method and avoid using interfering wavelengths between elements?
Autumn: The best way to do this is by running high purity, single element standards for each of the analytes in your method, monitor all wavelengths of interest for the method (multiple wavelengths for all analytes) and using the results to identify any interferences on wavelengths of interest for the other analytes. The single element standards would be run vs. A calibration curve constructed for all analytes. Here is an example of an interference table for an analysis like this built from 5 single element standards with 6 monitored wavelengths:
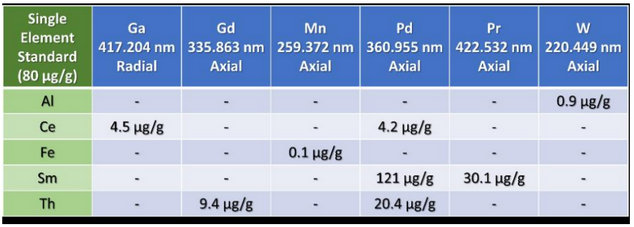
So, in this example, we know that Al will cause an interference on W 220.449 nm, so we’ll want to use a different wavelength for W.
Since we work with various environmental samples, both liquids and solids, what is the best way to correct for interfering elements?
Autumn: A very useful option we have used is to build an interference table for the method (See Previous Answer). This requires running high purity single element standards for each analyte vs. the multi element calibration curve for the method and monitoring all wavelengths of interest. In this way, you can see which elements will cause interferences or high bias on other elements, and at what level, based on the concentration of the major element. These interference corrections can then be applied to samples as long as peaks remain centered, and the calibration is current.
I’m new to ICP-OES. Regarding blockages of the nebulizer, how can one tell if it is a true blockage or if the issue is due to faulty valve switching?
Autumn: Pull the nebulizer out of the spray chamber and physically observe the mist to ensure it looks consistent and of the proper density.
Sergei: Additionally, try to bypass the valve and see if you get a good mist. If you see a difference, the problem is the blockage in the Valve itself or in the sample line adapter used by the Fast Valve manufacturers that has a very tiny ID of only 0.25 mm! If that is the case, I suggest to our customers change to a different sample line adapter with larger ID of 0.50 mm and make a minor change to the method for timing needed to deliver the sample from the Valve to the nebulizer.
What is the best solution for cleaning the nebulizer and tubing to prevent build up?
Autumn: Tubing should always be checked for any obvious signs of buildup, kinking, or damage and should be replaced at the first sign. You should also check that the fittings of the tubing to the nebulizer are tight and should replace them when they begin to fit too loosely. You should rinse your nebulizers frequently by backflushing with water, dilute acid, or a detergent solution (we use 25% RBS-25) at least weekly. You can also soak the nebulizers if they are especially dirty but NEVER sonicate and NEVER insert anything into the tip of the nebulizer. Follow soaking with thorough deionized water rinses. We generally recommend soaking nebulizers monthly, but this depends on use and sample types.
Can you test organics using a non-cooled spray chamber? We have only tested aqueous solutions prior to trying organics. We have been able to stabilize the plasma by lowering the sample flow and increasing the RF power and Argon flow, but we are not getting much of a signal from many of the elements we test for. I am not sure if the lack of cooling is the problem or our injector diameter.
Sergei: Yes, you CAN in general. But it seems you have done everything possible to reduce the sample load into the plasma and maintain it without shutdowns. Loss of sensitivity in this case is expected. If you are not reaching the needed detection power, then I am afraid a cooled spray chamber should be tried.
Autumn: Having a peltier cool spray chamber will greatly improve results especially for volatile organics. Cooling the spray chamber allows nebulization efficiency of the analyte to be controlled by the nebulizer flow and not by the form of analyte in the sample being aspirated (gaseous form of the volatile analyte would be transported to the plasma at a higher rate and cause high and inconsistent results, along with inconsistent background from other volatile components in the matrix).
I have an ICP-OES, and the samples I analyze are high in salt. I installed an argon humidifier approximately 6 months after the ICP-OES was installed. After a couple of months, I started getting an error of “Nebulizer channel has failed. Flow is outside of tolerance.” It was determined that the argon
humidifier was causing this error. I was told to remove the argon humidifier and purchase a new humidifier. Removing the humidifier did correct the problem, and I have not put the argon humidifier back in service. Is this a normal problem with the argon humidifier? Replacing the humidifier could become costly. Do you have any other recommendations?
Sergei: I am very sorry to hear about your experience. While the Ar humidifier is certainly a “must have” when running high salt samples, it is an additional component that introduces a potential for other problems if not installed or maintained correctly. It could have been just a simple connection issue that is easily fixable.
Autumn: The water in the Argon humidifier should be deionized. It’s also a good idea to change the water in the humidifier pretty regularly to make sure the water is fresh and clean. Also, the gas lines should be checked regularly for corrosion if the fittings are metallic and replace the fittings as needed. PEEK fittings would be better than metal if available.
We tend to run high TDS samples and noticed that the Silicon values were out of range. The torch was being etched by the samples. How can I reduce this?
Sergei: As mentioned during the presentation, try to increase the auxiliary gas flow in the torch to lift the plasma up away from the injector tip. If you are looking at really low Si values in the single PPB range, then a special sample intro might be needed.
Autumn: We recommend using a ceramic torch, a sapphire, alumina, or ceramic injector, HF-resistant (plastic) nebulizer and spray chamber, and a plastic elbow (if applicable) for your intro system. Any glass or quartz components will leach Si and B when caustic solutions are run through the system.
Could you please repeat the elements that glass is an issue for? I knew some of them were not great to use glass with, but others were new to me.
Sergei: Typical “suspects” from the Borosilicate glass are Silicon, Boron, Sodium. and Calcium.
Autumn: Elements and concentrations that we typically avoid glass packaging for are listed below. Keep in mind these are very conservative levels to keep uncertainty very low and certified values within a tight window.
Limit of packaging in glass (ug/mL):
Al – 45
Ba – 15
B – 35
Ca – 15
Ga – 0.5
Fe – 5
Ni – 5
K – 20
Na – 75
Sr – 0.5
Zn – 2
Zr – 0.5
